
1.0Iron (Fe): The Foundation of Structural Materials and a Vital Element for Life

1.1Physical and Chemical Properties:
- Metallic luster, silvery-gray appearance, hard yet ductile;
- High melting point (1539°C), suitable for high-temperature processing;
- A reactive metal, readily reacts with oxygen, water, and acids;
- Common oxidation states are +2 and +3.
1.2Main Applications:
Steelmaking:
Iron’s most important application is in the production of steel. Steel is an alloy of iron and carbon, often mixed with manganese, chromium, nickel, and other elements to improve strength, toughness, or corrosion resistance. Steel is widely used in:
- Structural construction (e.g., bridges, high-rise buildings)
- Machinery and equipment manufacturing
- Transportation (cars, ships, railways)
- Home appliances and everyday goods


Industrial Chemicals and Fertilizers:
Iron compounds are used to produce dyes, catalysts, water treatment agents, and iron-based fertilizers.
1.3Biological Role:
Iron is an essential trace element for living organisms. Its primary functions include:
- Constituting hemoglobin and myoglobin, involved in oxygen transport;
- Participating in mitochondrial energy metabolism;
- Serving as a component of various enzymes and proteins.
1.4Historical and Cultural Significance:
- Human use of iron dates back to the Iron Age (around 1200 BCE), when iron tools and weapons replaced bronze ones, significantly advancing agricultural production and military capability;
- The widespread adoption of iron smelting technology marked a key milestone in human civilization.
- To this day, iron remains the most widely used metallic material globally.
Densities of selected elements
| element | density (g/cm3) | appearance |
| aluminum | 2.70 | silvery white, metallic |
| antimony | 6.68 | silvery white, metallic |
| cadmium | 8.64 | silvery white, metallic |
| carbon (graphite) | 2.25 | black, dull |
| chromium | 7.2 | steel gray, hard |
| cobalt | 8.9 | silvery gray, metallic |
| Copper
Gold |
8.92
19.3 |
reddish, metallic
yellow, metallic |
| iron | 7.86 | silver, metallic |
| lead | 11.3 | silvery-bluish white, soft, metallic |
| manganese | 7.2 | gray pink, metallic |
| Nickel
Platinum |
8.9
21.4 |
silver, metallic
silver, metallic |
| silicon | 2.32 | steel gray, crystalline |
| silver | 10.5 | silver, metallic |
| tin (gray) | 5.75 | gray |
| tin (white) | 7.28 | white metallic |
| Zinc | 7.14 | bluish white, metallic |
2.0Understanding Density: Definition, Calculation, and Iron as an Example
2.1What Is Density?
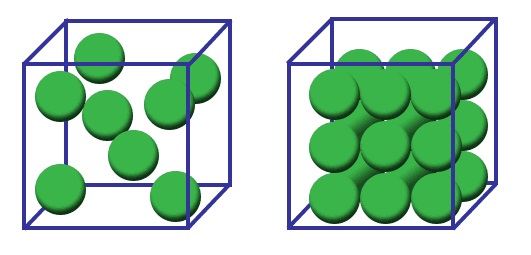
Common units of density include:
- SI unit: kilograms per cubic meter (kg/m³)
- Laboratory units: grams per cubic centimeter (g/cm³) or grams per milliliter (g/mL)
- Density is commonly represented by the Greek letter ρ (rho)
2.2Density Calculation Formula
Density (ρ) = Mass (m) / Volume (V)
Where:
- Mass is usually measured in grams (g)
- Volume can be expressed in milliliters (mL)or cubic centimeters (cm³)
(Note: 1 mL = 1 cm³)
2.3Example: Density of an Iron Block
An iron block has a mass of 23.6 grams, with dimensions of 2.0 cm × 2.0 cm × 0.75 cm. Determine its density and whether it is likely to be made of iron.
Volume = 2.0 × 2.0 × 0.75 = 3.0 cm³
Density = 23.6 g ÷ 3.0 cm³ = 7.87 g/cm³
Conclusion:
The object has a density of approximately 7.87 g/cm³, which is very close to the standard density of pure iron. Therefore, it is most likely pure iron or an iron-based alloy.
2.4Density of Iron and Iron Alloys
The density of pure iron is approximately 7.874 g/cm³
(or 491.5 lb/ft³, 0.284 lb/in³)
The table below lists the densities of common types of iron and iron alloys at room temperature. These values are useful for material selection and engineering calculations.
| Density of Iron and Iron Alloys | ||
| Material | Density | |
| g/cm3 | lbm / in3 | |
| Pure iron | 7.874 | 0.2845 |
| Ingot iron | 7.866 | 0.2842 |
| Wrought iron | 7.7 | 0.2 |
| Gray cast iron | 7.15 Note-1 | 0.258 Note-1 |
| Malleable iron | 7.27 Note-2 | 0.262 Note-2 |
| Ductile iron | 7.15 | 0.258 |
| High-nickel iron (Ni-Resist) | 7.5 | 0.271 |
| High-chromium white iron | 7.4 | 0.267 |
Note-1: 6.95 to 7.35 g/cm3 (0.251 to 0.265 lb/in.3).
Note-2: 7.20 to 7.34 g/cm3 (0.260 to 0.265 lb/in.3).
3.0Factors Affecting the Density of Iron
3.1Atomic Structure
The density of iron is influenced by its crystal structure:
- Body-Centered Cubic (BCC) Structure:Ferrite (α-iron, BCC) has a slightly lower density compared to austenite (γ-iron, FCC) due to its atomic packing efficiency.
- Face-Centered Cubic (FCC) Structure: Found in austenite (γ-iron), which has a higher density
3.2Temperature and Phase Transitions
As temperature increases, iron undergoes phase transitions that affect its crystal structure and thus its density:
- α-iron (BCC)transitions to γ-iron (FCC) at approximately 912°C
- γ-iron (FCC)transforms into δ-iron (BCC) at around 1394°C
- The melting pointof iron is approximately 1538°
3.3Addition of Alloying Elements
- Adding elements such as carbonalters the structure and density of iron
- For example, increasing carbon content in steel leads to the formation of pearlite, and lowers the phase transition temperatureto around 727°C.
4.0Frequently Asked Questions about the Density of Iron
4.1What is the density of pure iron?
The density of pure iron is approximately 7.874 g/cm³ (or 491.5 lb/ft³, 0.2845 lb/in³) at room temperature.
4.2Does the density of iron change with temperature?
Yes, the density of iron varies with temperature due to crystal structure phase transitions. For example, at 912°C, α-iron (BCC) becomes γ-iron (FCC), which has a slightly higher density.
4.3What affects the density of iron alloys like cast iron or ductile iron?
The density of iron alloys depends on the types and amounts of alloying elements (e.g., carbon, nickel, chromium) and the microstructure. For instance, gray cast iron has a lower density (~7.15 g/cm³) due to its graphite flakes and porosity.
4.4How is the density of iron calculated?
Density is calculated using the formula:
Density (ρ) = Mass (m) / Volume (V)
Example: A 23.6 g iron block with a volume of 3.0 cm³ has a density of 7.87 g/cm³.
4.5Why is iron’s density important in engineering?
Iron’s density directly affects material weight, structural load, and design feasibility. Engineers use density to choose materials for buildings, machinery, and transportation to balance strength, weight, and cost.
Other: Density of aluminum
References:
https://www.princeton.edu/~maelabs/mae324/glos324/iron.htm
https://web.fscj.edu/Milczanowski/psc/lect/Ch4/slide6.htm
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/iron-fertilizers


